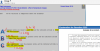
I'm prepping for my FAA Sport Pilot test by doing lots of practice tests. I like to think I understand the physics of temperature and pressure but I get pretty much every question related to these two items and the altimeter wrong. I've tried searching for help but the sites I find just confuse me more. The specific question is attached as a screenshot from the King Air training system.
What I think should happen: If temp goes up, air density goes down (molecules move faster and are further apart) which is why on hot days you need more runway to take off. It seems to me that if the temp goes up, the altimeter reading should also go up since it's a pressure measuring device and should be seeing a lower atmospheric pressure and display a higher altitude. WRONG. Per the attached screenshot, as temps go up, so does pressure??? If I have a closed system (tire, for instance) this makes sense since the air has no place to go and increased molecular motion results in higher pressure. I am thinking of the atmosphere as an essentially unbounded/unconstrained system so as the air expands it moves out in all directions resulting in lower pressure.
I don't get the mechanics of what's going on. Does anybody have a simple explanation for what happens and why? Is my assumption that the atmosphere is unbounded the problem? It IS a closed system much like a tire and acts the same way?0
Once I finally wrap my head around this one, I'll post another question about the relationship between air pressure and the altimeter. That one seems backward to me too...
Cannot wait to be done with the test...then I can start perceverating on the check-ride, specifically the oral...
What I think should happen: If temp goes up, air density goes down (molecules move faster and are further apart) which is why on hot days you need more runway to take off. It seems to me that if the temp goes up, the altimeter reading should also go up since it's a pressure measuring device and should be seeing a lower atmospheric pressure and display a higher altitude. WRONG. Per the attached screenshot, as temps go up, so does pressure??? If I have a closed system (tire, for instance) this makes sense since the air has no place to go and increased molecular motion results in higher pressure. I am thinking of the atmosphere as an essentially unbounded/unconstrained system so as the air expands it moves out in all directions resulting in lower pressure.
I don't get the mechanics of what's going on. Does anybody have a simple explanation for what happens and why? Is my assumption that the atmosphere is unbounded the problem? It IS a closed system much like a tire and acts the same way?0
Once I finally wrap my head around this one, I'll post another question about the relationship between air pressure and the altimeter. That one seems backward to me too...
Cannot wait to be done with the test...then I can start perceverating on the check-ride, specifically the oral...