S
skycaptainspeedbird
Guest
Is anyone asking the right questions? You're really going to have to want the answer, to read all of this.
The fuel being used on this Boeing 777-ER was Jet A-1 spec. confirmed by AAIB.
JET A-1 Fuel is:
Jet A-1 is a kerosene grade of fuel suitable for most turbine engined aircraft. It should, have a flash point above 38°C (100°F) and a freeze point of -47°C
Occidental Jet A1-Fuel normally contains, but is not required to contain:
Antioxidants to prevent gumming, (alkylated phenols)
Antistatic agents, to dissipate static electricity and prevent sparking;
Corrosion inhibitors, e.g. DCI-4A
Fuel System Icing Inhibitor (FSII) agents
Bacteriocidal "Biocide" agents
The fuel in BA038 wouldn't contain:
Biocide (Unlikely in the first place, in case of doubt, the fuel system was checked for water etc. Jan 15th, and it would have been added to the outbound fuel if necessary) [AAIB]
May have contained: (these are beneficial to the ground crew)
Antioxidants
Antistatic agents
FSSI-type Agents (we'll come back to this)
May have contained some types of these:
Corrosion inhibitors, e.g. DCI-4A (but, not the types needed!)
The AAIB pointed out the freezing point for the specific batch of fuel found in the plane's tanks was -57 C.
The fuel estimation system deduced, that the expected fuel needed to arrive at London Heathrow with 6,900 kg remaining would be 79,000 kg. Weight of the aircraft, contents, and weather, should already be factored in here. The aircraft left with 79,000 kg and the flight conditions were cold but not exceptional.
The plane arrived with an estimated 10,500 kg.
- At the high speeds that jet engines operate, the engine performance decreases with decreases of engine altitude and so, with increases of atmosphere pressure. - (to a point - "cruising altitude")
AAIB confirms that the plane flew higher than planned. We don't as yet have any specific information about the Thrust Specific Fuel Consumption (TSFC) [TSFC = FF/Thrust]; the TSFC, was obviously much lower than planned.
But Why?
Jet A-1 standards are not strict at all, read "Standards Requirements for Domestic and Foreign Jet Fuels for Civil Aviation" in the Journal of Chemistry and Technology of Fuels and Oils. What they discuss, are quality indices, for Jet Fuel. But isn't the Jet A-1 "strict" standard enough? Well these professors didn't think so.
NOW THIS GETS COMPLICATED IF YOU DON'T HAVE A CHEM BKGND DON'T GIVE UP:
Jet fuel much like gasoline, is a mix of hydrocarbon chains (hydrogen bonded with carbon -- we use the hydrogen, and expel the carbon as waste gas); these chains vary in length. Notice that you pay by the octane level in the gasoline? Oct = 8 for 8 carbons. Octane is the best fuel for high performance cars, unfortunately in the gasoline (PRODUCT NAME) you have longer or shorter carbon chains (+ or - Octane) to a very high degree, in fact petrol or gasoline is a combination of septane, octane, nonane, decane, and, well you see; there are a lot of ways to cheat, when a fuel is not branded by the exact chemicals it contains.
So what happens as these carbon numbers rise? Well methane, I think everyone knows is a gas, and it has 1 carbon and 4 hydrogens (C1 H4), and octane (C8 H18), I think everyone knows by now, is a liquid. The higher the number of carbons, the more hydrogen (energy) the fuel possesses, but the thicker, more dense, and harder to burn, the fuel becomes. It also raises the freezing point. So we need to find a balance between a long chain hydrocarbon so that we can get more thrust per litre or gallon and not make it solid at -47 C. Right!!
Jet fuel is based on kerosene, and should have hydrocarbons in it that vary in length from 10-16 carbons. Something like cetane C16 H34 (16 carbons 34 hydrogens) has immense energy per molecule and is barely liquid at room temperature, it freezes at around 17 C; decane freezes around (C10 H22) around -28 C.
Jet A-1 is partially made by changing the distillation cut points or component blending to produce a jet/aviation fuel having an acceptable freeze point. The desired freeze point being -47 C for Jet A-1. But decane still freezes around -28 C. How do we make it freeze at a lower temperature? Well, there are a lot of ways. There are thousands of fuel additives most ending in -ene that have very low freezing points, various polymers, are useful as "middle distillate pour point depressants" and are prepared from ethylene etc.
These pour depressants include copolymers of ethylene and vinyl esters of lower fatty acids such as vinyl acetate (U.S. Pat. No. 3,048,479); copolymers of ethylene and alkyl acrylate (Canadian Patent No. 676,875); terpolymers of ethylene with vinyl esters and alkyl fumarates (U.S. Pat. Nos. 3,304,261 and 3,341,309); polymers of ethylene (British Patent Nos. 848,777 and 993,744); chlorinated polyethylene (Belgian Patent No. 707,371 and U.S. Pat. No. 3,337,313.
Wait, wait, polymers and patents? That sounds expensive. It is.
-57 C degrees is pretty low even for a long haul in winter, that's more like Jet B fuel, except that we have extra fuel to think of. Oh, and don't forget that because jet fuel is a mixture of so many different fractions of hydrocarbons, and other chemicals, it remains pumpable for about ten degrees below the temperature where it starts to freeze.
Now what has a freezing point of -57 C? Wait for it...
Octane does!
Basically, they tried to run the plane on something much closer to petrol/gasoline than kerosene?
Yes, Jet fuel and Gasoline have about the same API Gravity. What does that mean? It means that one gallon of jet A fuel, and one gallon of gasoline weigh about the same, 6.76lbs per gallon and 6.73lbs per gallon respectively; well to me or you they do, but on a plane, those differences would be greatly magnified. Someone would have noticed the extra volume.
Oh wait but, there is no "basically", or anything basic, about a catastrophic failure like BA038.
And even if they were running on gasoline, they would not have had fuel left over. Extra fuel would suggest the opposite of everything we've been saying.
It gets much more complicated.
Oh that's right, we never talked about isomers of hydrocarbons. Oh, I hear your pain. C10 H8, ten carbons, shouldn't that be decane? Wait, why are there only eight carbons? Because the carbon molecules are holding on to each other differently, now there is less room for hydrogen to attach. Picture carbon with four arms, so one carbon can hold on to four molecules of hydrogen, hydrogen only has one arm. (C1 H4 Methane) So put two carbons next to each other, and one arm of each goes to the other carbon to keep them together, and the two of them now have 6 other free arms to grab a hydrogen (C2 H6 Ethane). Couldn't it just be that simple. No, God has a sense of humour. The carbons can hold on to other carbons with two or three arms and make funny shapes instead of neat rows. C10 H8 is the empirical formula for NAPHTHALENE check out this picture:
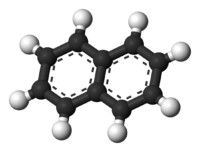
Ok, what the hell are you talking about now?
Remember I talked about "other chemicals" in fuel. Well, turns out China isn't as rich in oil as it is in coal. You can use coal to make gasoline, or kerosene, with one clue, unusual amounts of NAPHTHALENE residue or "Moth Balls" and if you've checked, coal is much cheaper than petroleum right now.
So what?
Well the properties of that fuel are going to be pretty different because of the "other chemicals" that are by-products of the fractional distillation of coal. It's not the same as the fractional distillation of petroleum; your going to have all these nasty crazy things called isomers, like napthalene, that make the jet fuel poor quality.
And the engine damage? Well diesel fuel, like gasoline and jet fuel, is rated as well, except not by octane number but, by cetane number (C16 H34). Cetane having a rating of 100 and Napthalene having a rating of 0.
Napthalene and other isomers of hydrocarbons cause engine damage by screwing with the fuel's ignition delay (the time period between the start of injection and start of combustion). But cause Cavitation, are you sure? Wait for it.
And the extra fuel? Well that is a question.
I'm in China, oil is at $100 a barrel but, I can make jet fuel from coal for almost nothing but, I'll have to add a few things to it to spruce it up. It doesn't matter how expensive they are, I'm still making a tonne of money.
What shall I add? What about the same stuff we add to diesel fuel on land, alkyl nitrates. Nitrogen products are part of the "other chemicals" that get produced when you distill oil or coal anyway, and we have all the right chemicals. Plus we'll add all the normal stuff for jet fuel, a dish of Xylene, and dash of Toulene, etc. but the fuel quality is still too low because we started with coal. Damn it.
Wait a second, I've got it, the key to our riches, I'll even add some of those high tech polymers. The money will roll in, and no one will be the wiser. Wish I knew more about polymers though.
What happens when you use high tech polymers as a fuel additives? Well it reduces the freezing temperature of the fuel quite a bit. Could i reduce cetane from freezing at 17 C to freezing at -57 C? Well not quite, but combined with more additives like xylene and toulene, sure, with out issue but, you have to handle polymers with care.
Why? because fuel is moved and pumped all over the plane. So? Movement causes foaming, that's where the antifreeze agents (the polymers), and the fuel separate especially in very cold weather. What happens when the polymers foam? It causes fuel pump cavitation.
Well that's a coincidence, G-YMMM had pump cavitation. Hmmm.... isn't it just.
Ok, so the fuel's hydrocarbon chain was longer than normal, but it met with A-1 Spec freezing temperature of -47 C in fact it exceeded by 10 C. That explains the fuel economy, the polymers explain the temperature rating, the absence of sufficient de-foaming agent explains the cavitation but, why did the fuel pumps fail?
They had simply had enough of pumping around this sludge, and they were not designed to do it either.
Wouldn't it be happening to other planes on other airlines. Not necessarily, the route of BA038 is unusual, the plane's fuel system is highly automated, this plane G-YMMM was a frequent Beijing visitor, each airline makes it's own fuel arrangements and, it may just not have happened yet. There are many, many, things that all have to go wrong for a failure like this to occur.
How do you know all this? Well nothing here is written in stone but, it's all in the science, my friend. Empirical evidence doesn't lie.
**Someone should pass this on to AAIB. They're probably going crazy trying to figure it out, it has been awhile**
The fuel being used on this Boeing 777-ER was Jet A-1 spec. confirmed by AAIB.
JET A-1 Fuel is:
Jet A-1 is a kerosene grade of fuel suitable for most turbine engined aircraft. It should, have a flash point above 38°C (100°F) and a freeze point of -47°C
Occidental Jet A1-Fuel normally contains, but is not required to contain:
Antioxidants to prevent gumming, (alkylated phenols)
Antistatic agents, to dissipate static electricity and prevent sparking;
Corrosion inhibitors, e.g. DCI-4A
Fuel System Icing Inhibitor (FSII) agents
Bacteriocidal "Biocide" agents
The fuel in BA038 wouldn't contain:
Biocide (Unlikely in the first place, in case of doubt, the fuel system was checked for water etc. Jan 15th, and it would have been added to the outbound fuel if necessary) [AAIB]
May have contained: (these are beneficial to the ground crew)
Antioxidants
Antistatic agents
FSSI-type Agents (we'll come back to this)
May have contained some types of these:
Corrosion inhibitors, e.g. DCI-4A (but, not the types needed!)
The AAIB pointed out the freezing point for the specific batch of fuel found in the plane's tanks was -57 C.
The fuel estimation system deduced, that the expected fuel needed to arrive at London Heathrow with 6,900 kg remaining would be 79,000 kg. Weight of the aircraft, contents, and weather, should already be factored in here. The aircraft left with 79,000 kg and the flight conditions were cold but not exceptional.
The plane arrived with an estimated 10,500 kg.
- At the high speeds that jet engines operate, the engine performance decreases with decreases of engine altitude and so, with increases of atmosphere pressure. - (to a point - "cruising altitude")
AAIB confirms that the plane flew higher than planned. We don't as yet have any specific information about the Thrust Specific Fuel Consumption (TSFC) [TSFC = FF/Thrust]; the TSFC, was obviously much lower than planned.
But Why?
Jet A-1 standards are not strict at all, read "Standards Requirements for Domestic and Foreign Jet Fuels for Civil Aviation" in the Journal of Chemistry and Technology of Fuels and Oils. What they discuss, are quality indices, for Jet Fuel. But isn't the Jet A-1 "strict" standard enough? Well these professors didn't think so.
NOW THIS GETS COMPLICATED IF YOU DON'T HAVE A CHEM BKGND DON'T GIVE UP:
Jet fuel much like gasoline, is a mix of hydrocarbon chains (hydrogen bonded with carbon -- we use the hydrogen, and expel the carbon as waste gas); these chains vary in length. Notice that you pay by the octane level in the gasoline? Oct = 8 for 8 carbons. Octane is the best fuel for high performance cars, unfortunately in the gasoline (PRODUCT NAME) you have longer or shorter carbon chains (+ or - Octane) to a very high degree, in fact petrol or gasoline is a combination of septane, octane, nonane, decane, and, well you see; there are a lot of ways to cheat, when a fuel is not branded by the exact chemicals it contains.
So what happens as these carbon numbers rise? Well methane, I think everyone knows is a gas, and it has 1 carbon and 4 hydrogens (C1 H4), and octane (C8 H18), I think everyone knows by now, is a liquid. The higher the number of carbons, the more hydrogen (energy) the fuel possesses, but the thicker, more dense, and harder to burn, the fuel becomes. It also raises the freezing point. So we need to find a balance between a long chain hydrocarbon so that we can get more thrust per litre or gallon and not make it solid at -47 C. Right!!
Jet fuel is based on kerosene, and should have hydrocarbons in it that vary in length from 10-16 carbons. Something like cetane C16 H34 (16 carbons 34 hydrogens) has immense energy per molecule and is barely liquid at room temperature, it freezes at around 17 C; decane freezes around (C10 H22) around -28 C.
Jet A-1 is partially made by changing the distillation cut points or component blending to produce a jet/aviation fuel having an acceptable freeze point. The desired freeze point being -47 C for Jet A-1. But decane still freezes around -28 C. How do we make it freeze at a lower temperature? Well, there are a lot of ways. There are thousands of fuel additives most ending in -ene that have very low freezing points, various polymers, are useful as "middle distillate pour point depressants" and are prepared from ethylene etc.
These pour depressants include copolymers of ethylene and vinyl esters of lower fatty acids such as vinyl acetate (U.S. Pat. No. 3,048,479); copolymers of ethylene and alkyl acrylate (Canadian Patent No. 676,875); terpolymers of ethylene with vinyl esters and alkyl fumarates (U.S. Pat. Nos. 3,304,261 and 3,341,309); polymers of ethylene (British Patent Nos. 848,777 and 993,744); chlorinated polyethylene (Belgian Patent No. 707,371 and U.S. Pat. No. 3,337,313.
Wait, wait, polymers and patents? That sounds expensive. It is.
-57 C degrees is pretty low even for a long haul in winter, that's more like Jet B fuel, except that we have extra fuel to think of. Oh, and don't forget that because jet fuel is a mixture of so many different fractions of hydrocarbons, and other chemicals, it remains pumpable for about ten degrees below the temperature where it starts to freeze.
Now what has a freezing point of -57 C? Wait for it...
Octane does!
Basically, they tried to run the plane on something much closer to petrol/gasoline than kerosene?
Yes, Jet fuel and Gasoline have about the same API Gravity. What does that mean? It means that one gallon of jet A fuel, and one gallon of gasoline weigh about the same, 6.76lbs per gallon and 6.73lbs per gallon respectively; well to me or you they do, but on a plane, those differences would be greatly magnified. Someone would have noticed the extra volume.
Oh wait but, there is no "basically", or anything basic, about a catastrophic failure like BA038.
And even if they were running on gasoline, they would not have had fuel left over. Extra fuel would suggest the opposite of everything we've been saying.
It gets much more complicated.
Oh that's right, we never talked about isomers of hydrocarbons. Oh, I hear your pain. C10 H8, ten carbons, shouldn't that be decane? Wait, why are there only eight carbons? Because the carbon molecules are holding on to each other differently, now there is less room for hydrogen to attach. Picture carbon with four arms, so one carbon can hold on to four molecules of hydrogen, hydrogen only has one arm. (C1 H4 Methane) So put two carbons next to each other, and one arm of each goes to the other carbon to keep them together, and the two of them now have 6 other free arms to grab a hydrogen (C2 H6 Ethane). Couldn't it just be that simple. No, God has a sense of humour. The carbons can hold on to other carbons with two or three arms and make funny shapes instead of neat rows. C10 H8 is the empirical formula for NAPHTHALENE check out this picture:

Ok, what the hell are you talking about now?
Remember I talked about "other chemicals" in fuel. Well, turns out China isn't as rich in oil as it is in coal. You can use coal to make gasoline, or kerosene, with one clue, unusual amounts of NAPHTHALENE residue or "Moth Balls" and if you've checked, coal is much cheaper than petroleum right now.
So what?
Well the properties of that fuel are going to be pretty different because of the "other chemicals" that are by-products of the fractional distillation of coal. It's not the same as the fractional distillation of petroleum; your going to have all these nasty crazy things called isomers, like napthalene, that make the jet fuel poor quality.
And the engine damage? Well diesel fuel, like gasoline and jet fuel, is rated as well, except not by octane number but, by cetane number (C16 H34). Cetane having a rating of 100 and Napthalene having a rating of 0.
Napthalene and other isomers of hydrocarbons cause engine damage by screwing with the fuel's ignition delay (the time period between the start of injection and start of combustion). But cause Cavitation, are you sure? Wait for it.
And the extra fuel? Well that is a question.
I'm in China, oil is at $100 a barrel but, I can make jet fuel from coal for almost nothing but, I'll have to add a few things to it to spruce it up. It doesn't matter how expensive they are, I'm still making a tonne of money.
What shall I add? What about the same stuff we add to diesel fuel on land, alkyl nitrates. Nitrogen products are part of the "other chemicals" that get produced when you distill oil or coal anyway, and we have all the right chemicals. Plus we'll add all the normal stuff for jet fuel, a dish of Xylene, and dash of Toulene, etc. but the fuel quality is still too low because we started with coal. Damn it.
Wait a second, I've got it, the key to our riches, I'll even add some of those high tech polymers. The money will roll in, and no one will be the wiser. Wish I knew more about polymers though.
What happens when you use high tech polymers as a fuel additives? Well it reduces the freezing temperature of the fuel quite a bit. Could i reduce cetane from freezing at 17 C to freezing at -57 C? Well not quite, but combined with more additives like xylene and toulene, sure, with out issue but, you have to handle polymers with care.
Why? because fuel is moved and pumped all over the plane. So? Movement causes foaming, that's where the antifreeze agents (the polymers), and the fuel separate especially in very cold weather. What happens when the polymers foam? It causes fuel pump cavitation.
Well that's a coincidence, G-YMMM had pump cavitation. Hmmm.... isn't it just.
Ok, so the fuel's hydrocarbon chain was longer than normal, but it met with A-1 Spec freezing temperature of -47 C in fact it exceeded by 10 C. That explains the fuel economy, the polymers explain the temperature rating, the absence of sufficient de-foaming agent explains the cavitation but, why did the fuel pumps fail?
They had simply had enough of pumping around this sludge, and they were not designed to do it either.
Wouldn't it be happening to other planes on other airlines. Not necessarily, the route of BA038 is unusual, the plane's fuel system is highly automated, this plane G-YMMM was a frequent Beijing visitor, each airline makes it's own fuel arrangements and, it may just not have happened yet. There are many, many, things that all have to go wrong for a failure like this to occur.
How do you know all this? Well nothing here is written in stone but, it's all in the science, my friend. Empirical evidence doesn't lie.
**Someone should pass this on to AAIB. They're probably going crazy trying to figure it out, it has been awhile**
Last edited by a moderator:

